Top posting users this month
| No user |
Latest topics
4 posters
Chemical Engineering.

Cephalo the Pod- Charles Babbage
- Posts : 1796
Reputation : 8
Join date : 2012-08-22
Age : 30
Location : Somewhere in London, jogging.
- Post n°1
 Chemical Engineering.
Chemical Engineering.
I'm gonna try and summarize the stuff I learned while enrolled in the Chemical Engineering program at Western University.

TheCryptKeeper- Wolfram Knight
- Posts : 2004
Reputation : 19
Join date : 2012-08-21
Age : 30
Location : 4 levels up and 1 room to the left
- Post n°2
 Re: Chemical Engineering.
Re: Chemical Engineering.
no pressure or nuthin

lil' poopie boy- High Maester of Verra
- Posts : 1438
Reputation : 22
Join date : 2012-08-21
Age : 30
Location : alone in the snow
- Post n°3
 Re: Chemical Engineering.
Re: Chemical Engineering.
chem tho

TheCryptKeeper- Wolfram Knight
- Posts : 2004
Reputation : 19
Join date : 2012-08-21
Age : 30
Location : 4 levels up and 1 room to the left
- Post n°4
 Re: Chemical Engineering.
Re: Chemical Engineering.
im so hyped for this!
i might have to do one myself. what topics would be sick?
programming?
security?
cryptography?
computability theory?
advanced algebra?
discrete math?
i might have to do one myself. what topics would be sick?
programming?
security?
cryptography?
computability theory?
advanced algebra?
discrete math?

Vyzor- Journeyman From the Far East
- Posts : 1010
Reputation : 19
Join date : 2012-08-21
Age : 30
Location : 43°15.5′N 81°08′W
- Post n°5
 Re: Chemical Engineering.
Re: Chemical Engineering.
I personally want to see programming, but thats just ME! 


TheCryptKeeper- Wolfram Knight
- Posts : 2004
Reputation : 19
Join date : 2012-08-21
Age : 30
Location : 4 levels up and 1 room to the left
- Post n°6
 Re: Chemical Engineering.
Re: Chemical Engineering.
programming could be LITTTT
i think i may do that.
except at the same time brandon, tyler, and trent all probably know the basics of programming already lol
but also all doing prog projects together or something just to learn and get better would be so fun
i think i may do that.
except at the same time brandon, tyler, and trent all probably know the basics of programming already lol
but also all doing prog projects together or something just to learn and get better would be so fun

Vyzor- Journeyman From the Far East
- Posts : 1010
Reputation : 19
Join date : 2012-08-21
Age : 30
Location : 43°15.5′N 81°08′W
- Post n°7
 Re: Chemical Engineering.
Re: Chemical Engineering.
YA I AGREE.

Cephalo the Pod- Charles Babbage
- Posts : 1796
Reputation : 8
Join date : 2012-08-22
Age : 30
Location : Somewhere in London, jogging.
- Post n°8
 Oh boy.
Oh boy.
So before I get into university-level stuff, I should make sure that we're all starting off with the same knowledge base, in case anyone has forgotten any fundamentals in the four years since we all graduated from high school (or maybe you never took/cared for high school chemistry).
Lesson 0 - High School Stuff (Part 1: Atoms and Elements)
Chemistry, as opposed to physics or biology, focuses on the differences between different kinds of matter: how they interact with one another and how they form in the first place.
For starters, let's take a gander at the most basic particle with which chemistry concerns itself; the atom.
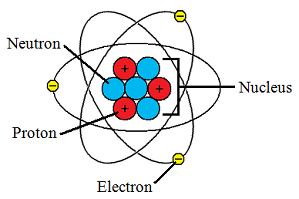
Pro-tip: every proton is the same. Every electron is the same. Every neutron is the same. Same mass, same charge. They only differ in how many other protons, neutrons and electrons to which they are connected.
Electrons have negative charge, protons have positive positive charge, and neutrons are neutral. Opposite charges bring particles together. Electrons have much less mass than protons and neutrons (the latter two are about equal, being about 2000 times larger than the former), so electrons are neglible when calculating an atom's mass.
Defining an atom relies mainly on the number of protons it has. The numbers of neutrons and electrons in an atom tend to grow with the number of protons. Electrons leave an atom much, much more easily than protons and neutrons do. This leaves the number of protons as the best tool for categorizing atoms.
Take a look at the periodic table:

Each square refers to a different element. An element is a substance which consists of atoms that all contain the same number of protons. The number at the top of each square on a periodic table is the atomic number, the number of protons in each atom. The number at the bottom is the atomic mass, which is pretty much equivalent to the total number of protons and neutrons in the average atom of the element (atoms of the same element differ slightly in the number of neutrons contained).
These elements form the basis for all chemical interaction.
Lesson 0 - High School Stuff (Part 1: Atoms and Elements)
Chemistry, as opposed to physics or biology, focuses on the differences between different kinds of matter: how they interact with one another and how they form in the first place.
For starters, let's take a gander at the most basic particle with which chemistry concerns itself; the atom.

Pro-tip: every proton is the same. Every electron is the same. Every neutron is the same. Same mass, same charge. They only differ in how many other protons, neutrons and electrons to which they are connected.
Electrons have negative charge, protons have positive positive charge, and neutrons are neutral. Opposite charges bring particles together. Electrons have much less mass than protons and neutrons (the latter two are about equal, being about 2000 times larger than the former), so electrons are neglible when calculating an atom's mass.
Defining an atom relies mainly on the number of protons it has. The numbers of neutrons and electrons in an atom tend to grow with the number of protons. Electrons leave an atom much, much more easily than protons and neutrons do. This leaves the number of protons as the best tool for categorizing atoms.
Take a look at the periodic table:

Each square refers to a different element. An element is a substance which consists of atoms that all contain the same number of protons. The number at the top of each square on a periodic table is the atomic number, the number of protons in each atom. The number at the bottom is the atomic mass, which is pretty much equivalent to the total number of protons and neutrons in the average atom of the element (atoms of the same element differ slightly in the number of neutrons contained).
These elements form the basis for all chemical interaction.

Cephalo the Pod- Charles Babbage
- Posts : 1796
Reputation : 8
Join date : 2012-08-22
Age : 30
Location : Somewhere in London, jogging.
- Post n°9
 Chemistry.
Chemistry.
Lesson 0 - High School Stuff (Part 2: Electron Arrangement and Chemical Bonding)
Atoms want to be electrically neutral; they want their protons and electrons to be equal in number. However, atoms also store their electrons in special arrangements, akin to shelves, called electron shells. Spaces within these shells are called orbitals
Atoms prefer to have their orbitals either full or empty. The only shell that tends to be partly full is the outermost (valence) one. This leads an atom to either give away its own electrons or take away electrons from other atoms, whichever is the simpler solution to its partly-full-shell problem. This is what leaves atoms with charges, positive or negative. A charged atom is called an ion.
Ions of opposite charge attract one another and then form chemical bonds to stay together. If two elements are far apart on the periodic table (both horizontally and verticallly), their atoms will form much stronger bonds, ionic bonds. Elements closer together will form covalent bonds.
A collection of atoms bonded covalently is called a molecule. Atoms bonded together ionically don't really form distinct particles, they just create a crystal lattice stricture. A substance whose molecules/crystall lattice are/is the same is called a compound. A compound is a substance of multiple elements bonded together in the same proportions all throughout.
Atoms want to be electrically neutral; they want their protons and electrons to be equal in number. However, atoms also store their electrons in special arrangements, akin to shelves, called electron shells. Spaces within these shells are called orbitals
Atoms prefer to have their orbitals either full or empty. The only shell that tends to be partly full is the outermost (valence) one. This leads an atom to either give away its own electrons or take away electrons from other atoms, whichever is the simpler solution to its partly-full-shell problem. This is what leaves atoms with charges, positive or negative. A charged atom is called an ion.
Ions of opposite charge attract one another and then form chemical bonds to stay together. If two elements are far apart on the periodic table (both horizontally and verticallly), their atoms will form much stronger bonds, ionic bonds. Elements closer together will form covalent bonds.
A collection of atoms bonded covalently is called a molecule. Atoms bonded together ionically don't really form distinct particles, they just create a crystal lattice stricture. A substance whose molecules/crystall lattice are/is the same is called a compound. A compound is a substance of multiple elements bonded together in the same proportions all throughout.

TheCryptKeeper- Wolfram Knight
- Posts : 2004
Reputation : 19
Join date : 2012-08-21
Age : 30
Location : 4 levels up and 1 room to the left
- Post n°10
 Re: Chemical Engineering.
Re: Chemical Engineering.
wow i already learned something that i didn't know i didn't know; the difference between a compound and a molecule.

Vyzor- Journeyman From the Far East
- Posts : 1010
Reputation : 19
Join date : 2012-08-21
Age : 30
Location : 43°15.5′N 81°08′W
- Post n°11
 Re: Chemical Engineering.
Re: Chemical Engineering.
Hey, I've always wondered, what's with Ununseptium and Ununoctium?

Cephalo the Pod- Charles Babbage
- Posts : 1796
Reputation : 8
Join date : 2012-08-22
Age : 30
Location : Somewhere in London, jogging.
- Post n°12
 Questions #117 and 118.
Questions #117 and 118.
Synthetic elements (they never occur in nature, too heavy) that have been created (in small amounts) but never named. The names refer to their atomic numbers: un-un-sept-ium is 117, un-un-oct-ium is 118.Vyzor wrote:Hey, I've always wondered, what's with Ununseptium and Ununoctium?
Around the 90s, elements are primarily created by mankind. They get named after places and people. Element 111 used to be unununium before being named roentgenium.

Vyzor- Journeyman From the Far East
- Posts : 1010
Reputation : 19
Join date : 2012-08-21
Age : 30
Location : 43°15.5′N 81°08′W
- Post n°13
 Re: Chemical Engineering.
Re: Chemical Engineering.
Cephalo the Pod wrote:Synthetic elements (they never occur in nature, too heavy) that have been created (in small amounts) but never named. The names refer to their atomic numbers: un-un-sept-ium is 117, un-un-oct-ium is 118.Vyzor wrote:Hey, I've always wondered, what's with Ununseptium and Ununoctium?
Around the 90s, elements are primarily created by mankind. They get named after places and people. Element 111 used to be unununium before being named roentgenium.
Oh ok, but how come they have no atomic mass? Is it because not enough have been created to even determine that number?

Cephalo the Pod- Charles Babbage
- Posts : 1796
Reputation : 8
Join date : 2012-08-22
Age : 30
Location : Somewhere in London, jogging.
- Post n°14
 Moles up the ass.
Moles up the ass.
Heads-up: in this post, I eventually start replacing "grams" with "g".
Lesson 0 - High School Stuff (Part 3: Moles and Molar Concentration)
Chemical reactions take place between individual atoms. It's not about how heavy the substances are, it's about how many particles you have. But counting individual atoms and molecules all the time would be impossible, so what we do is we use a shorthand.
Imagine a typical atom of carbon; 6 protons; 6 neutrons, leading to an atomic mass of 12. We already came up with a standard unit for mass; the gram. So we want a term to refer to how many atoms we need to get us 12 grams of carbon.
You can do the same for any element. Check the bottom of any square on the periodic table. That is the element's average atomic mass. A typical chlorine atom has either 35 or 36 protons+neutrons in its nucleus; how many atoms do you need to get somewhere between 35 and 36 grams of chlorine?
A mole is the number of atoms that gets you x grams of an element (where x is the average of the typical numbers of particles in the atom's nucleus). After some experiments, it turns out that a mole is equal to about 6.02x10^23 particles (imagine about 600 marbles. Multiply that by a thousand. Then multiply it by a billion. Multiply it by a billion again. That is a mole).
Here's where this becomes useful. Say you want to make some sodium chloride (table salt). You have some elemental sodium (a metal) and some chlorine (a poisonous gas). You don't want any leftover sodium or choroine. With a periodic table handy, you know to take 22.99g of sodium and 35.45g of chlorine. That means you have 6.02x10^23 atoms of sodium colliding with 6.02x10^23 atoms of chlorine to make 6.02x10^23 bonds, arranged in a crystal lattice structure. A mole of sodium chloride (NaCl) has a mass of 58.44g.
If you want 1000g of NaCl, you divide your desired mass by the molar mass of NaCl (calculations are approximate).
(1000g)/(58.44g/mol)=17.11mol
(17.11mol)*(22.99g/mol)=393.4g (you need about 393.4g of sodium).
(17.11mol)*(35.45g/mol)=606.6g (you need about 606.6g of chlorine).
The chlorine is heavier than the sodium, but it is completely used up in the reaction. This is why moles and molar mass are important.
Oh, and in addition: a lot of reactions take place when substances are dissolved in water. This makes molar concentration (moles/volume) another important measurement.
Lesson 0 - High School Stuff (Part 3: Moles and Molar Concentration)
Chemical reactions take place between individual atoms. It's not about how heavy the substances are, it's about how many particles you have. But counting individual atoms and molecules all the time would be impossible, so what we do is we use a shorthand.
Imagine a typical atom of carbon; 6 protons; 6 neutrons, leading to an atomic mass of 12. We already came up with a standard unit for mass; the gram. So we want a term to refer to how many atoms we need to get us 12 grams of carbon.
You can do the same for any element. Check the bottom of any square on the periodic table. That is the element's average atomic mass. A typical chlorine atom has either 35 or 36 protons+neutrons in its nucleus; how many atoms do you need to get somewhere between 35 and 36 grams of chlorine?
A mole is the number of atoms that gets you x grams of an element (where x is the average of the typical numbers of particles in the atom's nucleus). After some experiments, it turns out that a mole is equal to about 6.02x10^23 particles (imagine about 600 marbles. Multiply that by a thousand. Then multiply it by a billion. Multiply it by a billion again. That is a mole).
Here's where this becomes useful. Say you want to make some sodium chloride (table salt). You have some elemental sodium (a metal) and some chlorine (a poisonous gas). You don't want any leftover sodium or choroine. With a periodic table handy, you know to take 22.99g of sodium and 35.45g of chlorine. That means you have 6.02x10^23 atoms of sodium colliding with 6.02x10^23 atoms of chlorine to make 6.02x10^23 bonds, arranged in a crystal lattice structure. A mole of sodium chloride (NaCl) has a mass of 58.44g.
If you want 1000g of NaCl, you divide your desired mass by the molar mass of NaCl (calculations are approximate).
(1000g)/(58.44g/mol)=17.11mol
(17.11mol)*(22.99g/mol)=393.4g (you need about 393.4g of sodium).
(17.11mol)*(35.45g/mol)=606.6g (you need about 606.6g of chlorine).
The chlorine is heavier than the sodium, but it is completely used up in the reaction. This is why moles and molar mass are important.
Oh, and in addition: a lot of reactions take place when substances are dissolved in water. This makes molar concentration (moles/volume) another important measurement.
Last edited by Cephalo the Pod on Thu 11 Aug 2016, 5:08 pm; edited 1 time in total

Cephalo the Pod- Charles Babbage
- Posts : 1796
Reputation : 8
Join date : 2012-08-22
Age : 30
Location : Somewhere in London, jogging.
- Post n°15
 ...oh!
...oh!
I guess they haven't. Or maybe didn't, when this version of the table was made. It's possible that elements 117 and 118 were merely theoretical at the time.Vyzor wrote:Oh ok, but how come they have no atomic mass? Is it because not enough have been created to even determine that number?Cephalo the Pod wrote:Synthetic elements (they never occur in nature, too heavy) that have been created (in small amounts) but never named. The names refer to their atomic numbers: un-un-sept-ium is 117, un-un-oct-ium is 118.Vyzor wrote:Hey, I've always wondered, what's with Ununseptium and Ununoctium?
Around the 90s, elements are primarily created by mankind. They get named after places and people. Element 111 used to be unununium before being named roentgenium.

TheCryptKeeper- Wolfram Knight
- Posts : 2004
Reputation : 19
Join date : 2012-08-21
Age : 30
Location : 4 levels up and 1 room to the left
- Post n°16
 Re: Chemical Engineering.
Re: Chemical Engineering.
good stuff. allan wants to know what type of chem eng you're in

Cephalo the Pod- Charles Babbage
- Posts : 1796
Reputation : 8
Join date : 2012-08-22
Age : 30
Location : Somewhere in London, jogging.
General Chemical. As opposed to Biochemical (the split happens starting 3rd year). Biochemical students don't get as many technical electives as General Chemical, instead getting several biochemical courses. Other than that, we take the same stuff.lostboi_626 wrote:good stuff. allan wants to know what type of chem eng you're in

TheCryptKeeper- Wolfram Knight
- Posts : 2004
Reputation : 19
Join date : 2012-08-21
Age : 30
Location : 4 levels up and 1 room to the left
- Post n°18
 Re: Chemical Engineering.
Re: Chemical Engineering.
word, lovin these updates too

Cephalo the Pod- Charles Babbage
- Posts : 1796
Reputation : 8
Join date : 2012-08-22
Age : 30
Location : Somewhere in London, jogging.
- Post n°19
 Gassy.
Gassy.
Lesson 0 - High School Stuff (Part 4: Ideal Gas Law)
There are four important variables that must be observed when describing the state of a gas:
These variables change in proportion to one another. Temperature increases (or decreases) as pressure increases (or decreases); and does the same with volume (when the container can stretch). Volume increases when pressure decreases, and vice versa.
Here's that paragraph in equations:
In addition, when you add gas particles to a container, either pressure or volume increases. If you involve all four variables, you don't even need a second set of variables. You can just use one equation to compare pressure and volume to temperature and amount.
Here's the Ideal Gas Equation:
P*V=n*R*T
n is the number of moles of gas. R is an experimentally derived constant that matches the right side of the equation with the left in terms of both number and units. R is equal to about 8.314J/(mol*K). You can check the spoiler tag for an explanation in case you're confused by the units.
Now, this is called ideal for a reason. This equation doesn't account for internal attraction between gas molecules. In reality, the right side of the equation is typically multiplied by another dimensionless constant). We'll get to that when Thermodynamics II rolls around. Eventually. Very eventually.
There are four important variables that must be observed when describing the state of a gas:
- Pressure
- Volume
- Temperature
- Amount
These variables change in proportion to one another. Temperature increases (or decreases) as pressure increases (or decreases); and does the same with volume (when the container can stretch). Volume increases when pressure decreases, and vice versa.
Here's that paragraph in equations:
- (P1)/(T1)=(P2)/(T2)
- (V1)/(T1)=(V2)/(T2)
- (P1)*(V1)=(P2)*(V2)
In addition, when you add gas particles to a container, either pressure or volume increases. If you involve all four variables, you don't even need a second set of variables. You can just use one equation to compare pressure and volume to temperature and amount.
Here's the Ideal Gas Equation:
P*V=n*R*T
n is the number of moles of gas. R is an experimentally derived constant that matches the right side of the equation with the left in terms of both number and units. R is equal to about 8.314J/(mol*K). You can check the spoiler tag for an explanation in case you're confused by the units.
- Spoiler:
- Let's use SI units (m^3, Pa, J, mol, K, etc.)
Pressure is force/area. When you multiply by volume (which is area*(an extra dimension length)), you get force*length, which is equivalent to energy. The SI unit for energy is joule, J. It's equivalent to kg*(m^2)/(s^2).
Multiplying moles by temperature doesn't really get you anything meaningful. The constant needs to get mol*K to equal J. So the constant R has units of J/(mol*K).
Now, this is called ideal for a reason. This equation doesn't account for internal attraction between gas molecules. In reality, the right side of the equation is typically multiplied by another dimensionless constant). We'll get to that when Thermodynamics II rolls around. Eventually. Very eventually.

TheCryptKeeper- Wolfram Knight
- Posts : 2004
Reputation : 19
Join date : 2012-08-21
Age : 30
Location : 4 levels up and 1 room to the left
- Post n°20
 Re: Chemical Engineering.
Re: Chemical Engineering.
is there a reason it's called 'ideal'?
does it not always hold?
does it not always hold?

Cephalo the Pod- Charles Babbage
- Posts : 1796
Reputation : 8
Join date : 2012-08-22
Age : 30
Location : Somewhere in London, jogging.
- Post n°21
 PV=nZRT
PV=nZRT
See the last paragraph.lostboi_626 wrote:is there a reason it's called 'ideal'?
does it not always hold?
The Ideal equation does not account for attraction and repulsion between molecules. This alters the volume of the gas.

TheCryptKeeper- Wolfram Knight
- Posts : 2004
Reputation : 19
Join date : 2012-08-21
Age : 30
Location : 4 levels up and 1 room to the left
- Post n°22
 Re: Chemical Engineering.
Re: Chemical Engineering.
ahh okay sick

Cephalo the Pod- Charles Babbage
- Posts : 1796
Reputation : 8
Join date : 2012-08-22
Age : 30
Location : Somewhere in London, jogging.
Lesson 0 - High School Stuff (Part 5: Quantum Chemistry)
This part isn't too integral to Chemical Engineering, just helps you understand why certain atoms form certain numbers of bonds. For instance, a carbon atom forms four carbon bonds, an oxygen atom forms two, etc.
I've spoiler'd most of this post to emphasis my main point at the bottom.
For instance, a carbon atom will mix it's second shell's orbitals together to form four sp3 orbitals. The four electrons in the atom's second shell get distributed evenly. These four orbitals are what let the carbon atom form its four atomic bonds. This is what forms the basis of organic chemistry, and will be observed later.
This part isn't too integral to Chemical Engineering, just helps you understand why certain atoms form certain numbers of bonds. For instance, a carbon atom forms four carbon bonds, an oxygen atom forms two, etc.
I've spoiler'd most of this post to emphasis my main point at the bottom.
- Spoiler:
- First off, let me introduce you to four types of quantum numbers that help determine the position of an electrom relative to an atom.
- The principle atom number refers to the electron shell in which the atom is located. The 1st (innermost) shell is 0, the 2nd is 1, etc.
- The orbital quantum number refers to the type of orbital in which the atom is located. Within any one shell, the number of types of orbitals is equal to the number of the shell + 1. So in the 0 (1st) shell, there is only 1 kind of orbital, in the 1 (2nd) shell there are two types of orbitals. The first four types of orbitals are known as s, p, d, and f.
- The magnetic quantum number refers to which specific orbital in which the electron is located. The range of magnetic numbers an orbital of a certain type can have is equivalent to [-n...0...n], where n is the number of the electron shell in which that type of orbital is first found. Meaning, a p-orbital can have a magnetic number of -1, 0, or 1.
- The spin quantum number can, quite simply, be only +1/2 or -1/2.
Here's the thing about these numbers. There's this phenomenon known as the Pauli Exclusion Principle. Within any one atom, two electrons cannot share the same set of numbers. This is what determines the number of electrons in a shell.
In the first electron shell, there is only an s-orbital. There can only be one s-orbital, because the only magnetic quantum number an s-orbital can have is 0. In the second shell, there is one s-orbital and three p-orbitals. In the third shell, there is all of those, plus five d-orbitals.
There can be 1 orbital in the first shell, 4 in the second, 9 in the third, etc. And within each of these orbitals, two electrons can reside, because the two share different spin quantum numbers. So, there can be 2 electrons in the first shell, 8 in the second, 18 in the third, etc.
Now, look back to the periodic table. Its shape is very intentional. It's shaped in a way to illustrate how electron shells get filled. The first two columns show electrons going to s-orbitals. The short, middle rectangle is electrons going to d-orbirals. The right rectangle (except for helium) shows electrons going to p-orbitals. And the bottom rectangle shows electrons going to the f-orbitals.
Reading the table left to right, top to bottom shows the order of electron placement. The two electrons in the first shell get filled first. After that, the two electrons in the s-orbital of the second shell, and then the six electrons of the p-orbitals. And after that, the s-orbital and p-orbitals of the third shell get filled. The d-orbitals of the third shell get skipped over until after the 4th s-orbital. - The principle atom number refers to the electron shell in which the atom is located. The 1st (innermost) shell is 0, the 2nd is 1, etc.
For instance, a carbon atom will mix it's second shell's orbitals together to form four sp3 orbitals. The four electrons in the atom's second shell get distributed evenly. These four orbitals are what let the carbon atom form its four atomic bonds. This is what forms the basis of organic chemistry, and will be observed later.

TheCryptKeeper- Wolfram Knight
- Posts : 2004
Reputation : 19
Join date : 2012-08-21
Age : 30
Location : 4 levels up and 1 room to the left
- Post n°24
 Re: Chemical Engineering.
Re: Chemical Engineering.
cool i've never heard pauli exclusion principle explained in such simple terms

Cephalo the Pod- Charles Babbage
- Posts : 1796
Reputation : 8
Join date : 2012-08-22
Age : 30
Location : Somewhere in London, jogging.
- Post n°25
 Aw, geez, math on a keyboard.
Aw, geez, math on a keyboard.
Lesson 0 - High School Stuff (Part 6: Derivatives)
Chemical Engineering frequently involves equations that includes calculus. Nothing too complex, but it really helps to understand exactly what this stuff is.
You all remember the slope of a line, right? The ratio between the rise (change in y-value between two points) and the run (change in x-value between those same points)? Good.
Well lots of equations have non-straight graphs. The instantaneous slope at a specific point changes from point to point. We need to develop a method to find the derivative of a function. A derivative is an equation that can be used to find the instantaneous slope of another equation at any point.
So, here's what you do. You have two points for an equation. The first point has an x-value of, well, let's call it x, and a y-value of f(x). The second point has an x-value of (x+h) and a y-value of f(x+h). What you do, when you want to find the derivative, is you make h equal a number infinitely close to 0, so that you'll have two points infinitely close together.
The derivative is equal to
f(x+h)-f(x)
------------
(x+h)-x
Let's look at a specific example: x^2. The second y-value is (x+h)^2.
(x+h)^2-x^2
----------------
(x+h)-x
x^2+2*x*h+h^2-x^2
--------------------------
h
You end up with 2*x+h. But h practically equals 0. So you can simplify it to 2*x. Notation for generic derivative is dy/dx, or d[f(x)]/dx, or f'(x).
Try it with other equations. Here's a quick guide to some handy derivatives. If you have questions about these, I'll be happy to clarify.
Chemical Engineering frequently involves equations that includes calculus. Nothing too complex, but it really helps to understand exactly what this stuff is.
You all remember the slope of a line, right? The ratio between the rise (change in y-value between two points) and the run (change in x-value between those same points)? Good.
Well lots of equations have non-straight graphs. The instantaneous slope at a specific point changes from point to point. We need to develop a method to find the derivative of a function. A derivative is an equation that can be used to find the instantaneous slope of another equation at any point.
So, here's what you do. You have two points for an equation. The first point has an x-value of, well, let's call it x, and a y-value of f(x). The second point has an x-value of (x+h) and a y-value of f(x+h). What you do, when you want to find the derivative, is you make h equal a number infinitely close to 0, so that you'll have two points infinitely close together.
The derivative is equal to
f(x+h)-f(x)
------------
(x+h)-x
Let's look at a specific example: x^2. The second y-value is (x+h)^2.
(x+h)^2-x^2
----------------
(x+h)-x
x^2+2*x*h+h^2-x^2
--------------------------
h
You end up with 2*x+h. But h practically equals 0. So you can simplify it to 2*x. Notation for generic derivative is dy/dx, or d[f(x)]/dx, or f'(x).
Try it with other equations. Here's a quick guide to some handy derivatives. If you have questions about these, I'll be happy to clarify.
- Spoiler:
- d(x^n)/dx=n*x^(n-1) (where n is a constant)
- The derivative of a sum is the sum of derivatives.
- The derivative of a constant is 0, because it doesn't change.
- d[f(x)*g(x)]/dx=f'(x)*g(x)+g'(x)*f(x).
- d[f(x)/g(x)]/dx=[f'(x)*g(x)-g'(x)f(x)]/[g(x)]^2
- d{g[f(x)]}/dx=f'(x)*g'[f(x)]
- d[sin(x)]/dx=cos(x), d[cos(x)]/dx=-sin(x), d[tan(x)]/dx=1/[cos(x)]^2
- d(e^x)/dx=e^x, where e is Euler's number, about 2.7183
- d(a^x)/dx=(a^x)*ln(a), where a is a constant.
- d[ln(x)]/dx=1/x
- d(x^n)/dx=n*x^(n-1) (where n is a constant)
Last edited by Cephalo the Pod on Sat 27 Aug 2016, 12:02 am; edited 4 times in total


» "It's the door..... To the light" - The Social Thread v 2.0
» Diary of a Mac Student
» Arcasia: Legend of the Wolfram Emerald
» Le Cinéma
» Ideas
» Musique Lystoux
» Through Shady Lanes
» King of All Ratings Thread
» Where's The Beats!?